What We Learned at the 7th Supply Chain & Logistics Nexus Conference: Insights on the Biopharma Supply Chain
The biopharma industry has been under pressure for several years. Higher interest rates have made capital more expensive, forcing many companies to preserve cash and stretch runways while waiting for favorable clinical readouts to unlock new funding. Now, shifting regulatory and trade dynamics are adding another layer of uncertainty.
Against this backdrop, BioIT Solutions participated in the 7th Supply Chain & Logistics Nexus in San Francisco this May. Our goal: engage with supply chain professionals and better understand their top challenges.
When asked to name their biggest obstacle, one answer stood out:
“Coordinating with external partners and CMOs.”
That response is no surprise. Most emerging biotech companies outsource all or part of their manufacturing, and this trend has become the norm over the past two decades. Jed Brown, a veteran of Gilead, BeiGene, and Acerta, summed it up well:
“There are emerging pressures on global manufacturing production as an increase in approved biologics, including the new GLP-1 polypeptides drugs, soak up existing capacity. Combined with the diversified production demands of newer therapies (i.e., CGT, ADCs), this may trigger a shift back toward insourcing when the production scale and/or production flexibility cannot be adequately met by CDMOs.”
– Jed Brown, BioPharma Supply Chain Leader
Another major concern? Disruptions in supply or demand.
The March 2025 fire near Heathrow Airport caused a temporary shutdown that tested business continuity plans across the industry. Bassem Gayed, Senior Director of Technical Opeations at BMS, described how his team executed a rapid-response plan to deliver a batch of life-saving medicine to a critically ill patient despite the chaos.
Such cases underscore the importance of planning for logistics under stress.
Umar Hayat, Ph.D., VP of CMC and Supply Chain at UNION Therapeutics, shared practical strategies in his talk on “Derisking the Clinical Supply Chain.” One standout tip: Perform a “Dry Run” for clinical trial logistics. These test runs can expose unexpected permitting, customs, or temperature-control issues before they derail your timelines.
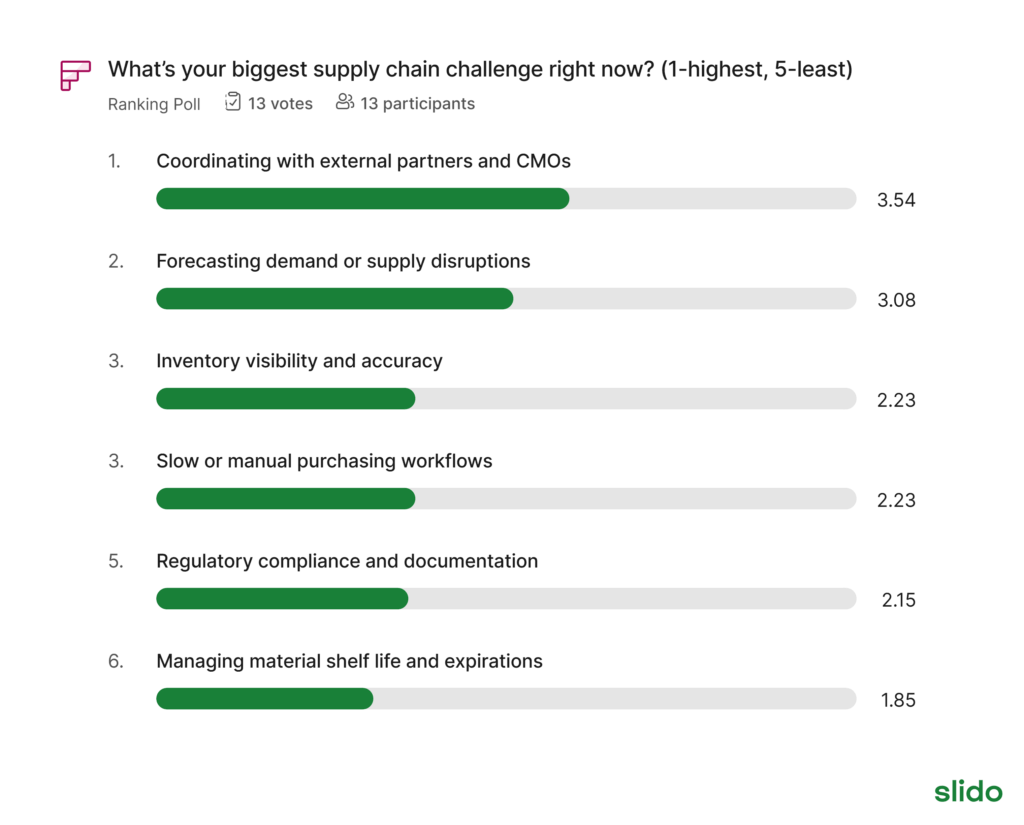
Interestingly, more routine operational issues—like inventory visibility, regulatory compliance, and shelf-life tracking ranked lower in urgency.
One thing is clear: Biopharma will bounce back.
With breakthroughs in gene and cell therapy, antibody-drug conjugates (ADCs), multi-antigen vaccines, and gene-editing on the horizon, the future remains bright. Investors and innovators who solve the coordination, forecasting, and resiliency issues highlighted here will be poised to lead the next wave of biotech success.
If you didn’t make it to the Nexus conference this year, contact us at sales@bioit.com or visit and follow us on LinkedIn for more insights and updates.
🚀 Ready to Tackle Your Supply Chain Challenges?
Choose your next step:
✅ Get the Free Implementation Kit – Our free Implementation Kit includes data import templates, implementation checklists, and project plans designed for emerging biopharma companies.
🤝 Book a 1:1 Strategy Session – Speak with a BioIT expert about your specific supply chain needs.
🎥 Coming Soon: Explore our interactive Demo Gallery with short videos on how our platform helps biotech teams digitize operations.

