Quality Assurance occupies a pivotal role in modern pharmaceutical companies because they sit at the nexus of production and clinical operations. From their perch, they have a birds-eye view of trends affecting their company and the industry at large. With this as backdrop, BioIT Solutions recently conducted a survey at the Society of Quality Assurance (SQA) 40th Annual Meeting and Quality College in Aurora, Colorado that asked attendees to weigh in on trends affecting QA.
To collect the data, we asked participants who visited the BioIT Solutions booth to fill out a 1-Question survey that asked, “What is the most consequential trend facing Quality Assurance Professionals?”. This free-form approach was designed to minimize influence and allow open-ended ideation. Later, we categorized the answers and present those findings here. The participants were all QA professionals ranging from senior level or department heads, industry consultants, and QA Operations, spanning GLP, GCP, and GMP.
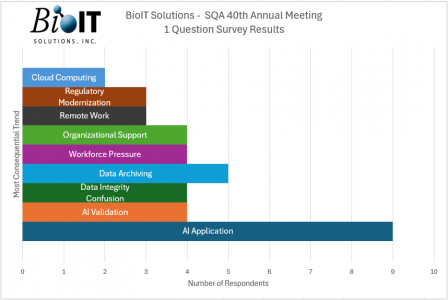
Predictably, Artificial Intelligence (AI), and Machine Learning (ML) were frequently mentioned. We heard interesting use cases that portend great leaps in productivity and compliance. Santosh Tharkude from Johnson and Johnson told how ML could be applied to production data to shorten batch release time and Joseph Whittemore from BeiGene outlined how he had been involved in a project that trained AI to recognize document properties in a vast document trove to aid their archival efforts.
Another AI/ML related topic was AI Validation. During one interactive session, attendees were asked to conceptually prepare for an AI product audit and were grouped into teams to collaborate. A couple of themes emerged, including the need for human involvement to ward off AI hallucinations and to always apply risk-based evaluations when offloading tasks to AI/ML. Together, AI applications (18%) and AI validation (9%) accounted for more than a quarter of the responses.
Also on the minds of respondents were the economic difficulties facing the pharmaceutical industry. They listed Workforce Pressure (14%) as being a notable headwind. We’ve all noticed the headlines indicating firms are conserving cash and delaying hiring or even resorting to layoffs. The Fierce Biotech Layoff Tracker shows no sign of an industry turnaround with 16 and 18 companies reporting staff reductions in February and March 2024 respectively. This leaves the remaining staff overburdened.
Another stressor is related to workforce turnover. Vinay Parachuri from PharmTek Solutions noted ”Retirement of qualified professionals and lack of experience in the new QA hires” as being a somewhat alarming trend. The rise of remote work, noted by 5% of responders, has limited mentoring opportunities and increased the need to formalize and automate QA processes. Until these improvements are adopted, the near future is cloudy because the current QA workforce is tasked with maintaining standards with reduced staffing.
Organizational Support (14%) was also mentioned frequently. Gabby Cook of XGen Pharma struck an optimistic tone when she identified “Getting buy-in for the quality culture throughout the entire company” as her stand-out trend. C-suite execs have learned that focusing on quality reduces waste, speeds turnaround times, and increases safety. And this has added heft to the quality message. Moreover, regulatory agencies are holding companies accountable for failure to meet their obligations. While the Federal Drug Administration rarely fines companies for failing to submit clinical trial data, Light Sciences Oncology became the fifth organization to receive a letter of noncompliance.
The respondents noted that the volume of information continues to grow, and this presents organizational challenges particularly around:
- Data Archiving (12%)
- Data Integrity Confusion (9%).
The continued reliance on paper records appears to be driving a lot of this confusion. Although methods exist to scan and archive documents, the volume is overwhelming for many respondents. We recommend leveraging AI/ML to tier records and implement automated lifecycle management. This will minimize your exposure to data breaches, damage and/or loss while saving time and improving your teams’ efficiency. SQA’s Computer Validation and IT Compliance (CVIC) specialty section is finalizing a data integrity handbook that should also help allay data integrity confusion.
Rounding out the trending categories were:
- Regulatory Modernization (7%),
- Cloud Computing (5%)
- Rapid Innovation (7%).
There is a good deal of interdependency between these categories since cloud computing and rapid innovation are prompting the agencies to modernize guidance. Cloud computing and the widespread availability of cloud-based systems (eQMS, LIMS, ERP) was mentioned by multiple respondents. This trend is especially exciting because there is no longer a need to procure and install servers and maintain a data center. In addition, the Software-As-A-Service (SAAS) model provides access to the latest software features and best practices. This makes enterprise computing possible for smaller companies that don’t have the resources or IT personnel needed to maintain computing centers.
Respondents outlined an exciting near-term future for the Quality Assurance industry. As advances in technology continue to offset the challenges posed by workforce turnover and financial headwinds. BioIT Solutions would like to thank all the participants who stopped by to participate in the survey.
| Why BioIT Solutions? BioIT Solutions is the “Digital On Ramp” for emerging biotech. We combine multiple functions into one unified system thereby amplifying data value and promoting collaboration. Our seasoned professionals are experts at helping biotech companies commercialize. If you’ve been searching for an easy to implement and easy to use system that meets the demands of both operations and regulators alike, you’ll be delighted with 1Platform4®. To learn more and explore working with us, please visit our website (https://bioit.com/wordpress) or email us (sales@bioit.com). |